The aim of establishing the Slovak Medicines Verification Organization is to develop
and implement an effective system for verifying the authenticity of medicines in the Slovak Republic, which will not be operated for the purpose of achieving or
generating a commercial profit, while the costs of operating this system will be borne by the holders of authorization for the production of medicines provided
protective elements.
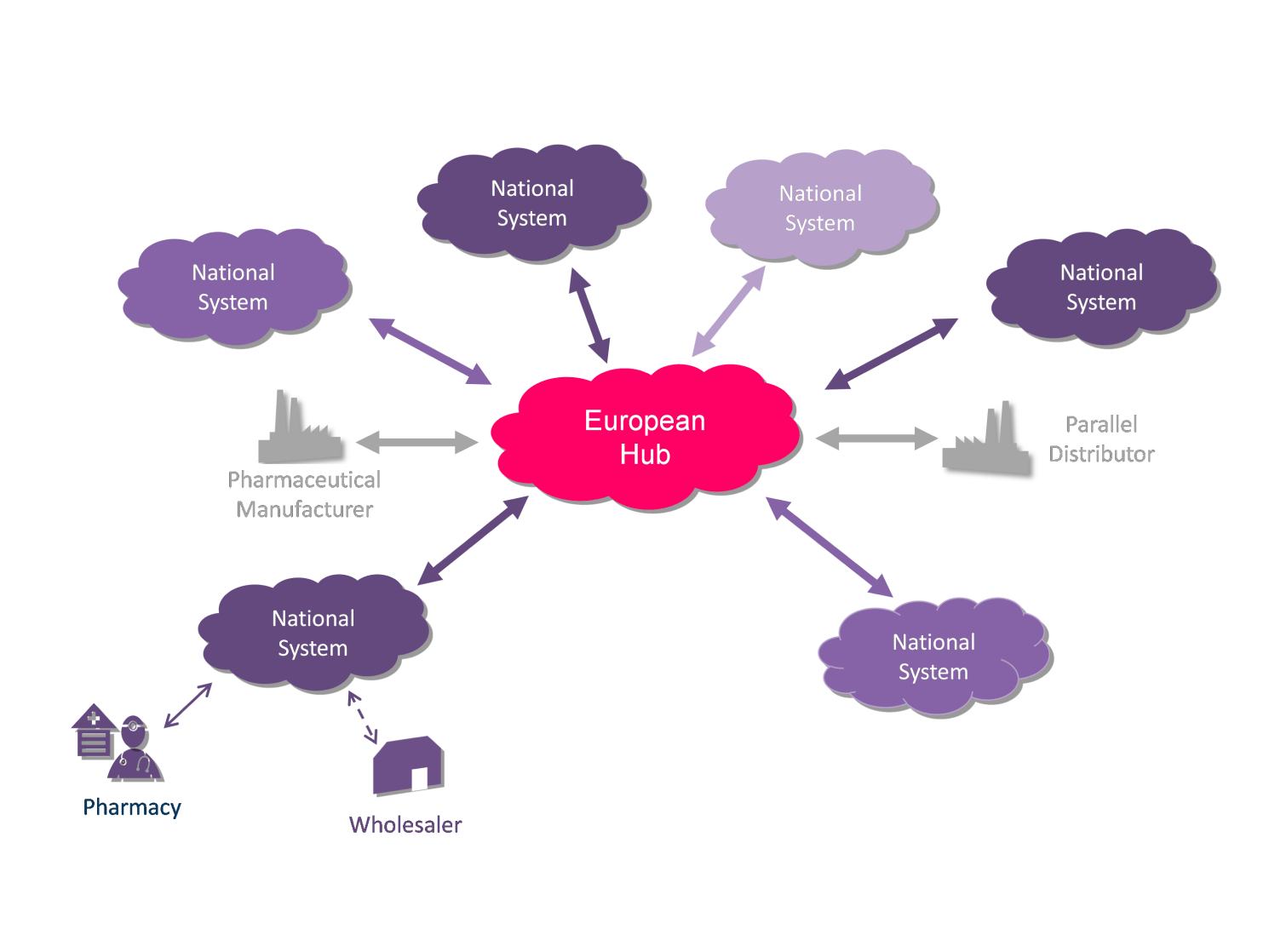
The aim of establishing the Slovak Medicines Verification Organization is also to create a comprehensive framework for the implementation of Directive 2011/62/EU, which will determine, among other things, access to data, control conditions and operational rules of the National Register, which will be connected to the Central and other National Registers in within the EU member states, which will create a Registration System, whereby within this platform they will
the participants of the pharmaceutical chain can verify the authenticity of each drug, while the Registration System will be interoperable between individual Member States and will work in relation to all drugs with the obligation to be equipped with protective elements.