What the Distributor must do to join the National Verification System:
* only distributors who distribute medicinal products for human use covered by the legislation in Commission Delegated Regulation (EU) 2016/161 are obliged to join the National Verification System.
- The distributor must have a valid permit for the distribution of human medicines in Slovakia, issued by the State Institute for Drug Control (ŠUKL).
-> ŠUKL must add this entity to the register on the e-vuc.sk portal and on this basis the entity will be assigned access data to the DISTRIBUTOR application.
-> The app is available at: https: //distributor.e-vuc.sk/ - The Distributor must log in to the DISTRIBUTOR application at: https: //distributor.e-vuc.sk/
- After logging into the application, you need to click on the Name of your Distribution Company in the left menu and click on the SOOL link in the top bar when it appears.
-> Then you need to click on the EDIT link and fill in all the required information.
-> After saving, the system will notify you to agree to the terms of use of the system and binding confirmation of registration.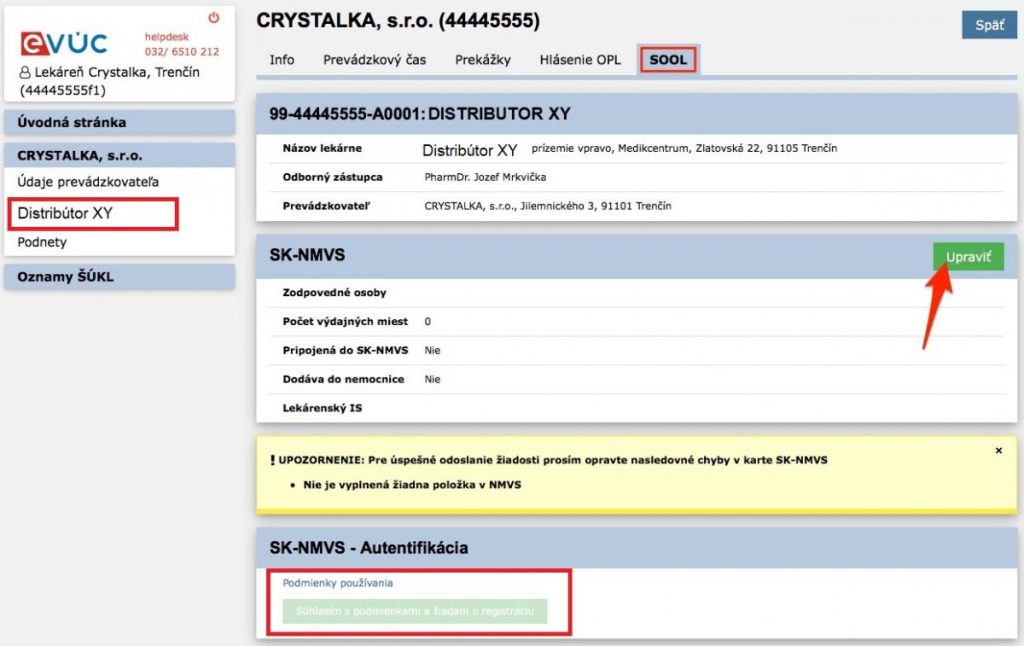
- SOOL will check the registration and create a user profile in the National Verification System within a few days.
- The access data to connect your software to the National Verification System will be sent by email to the address that was filled in when you registered in the Distributor application.
-> Your login details will also be recorded in your application at: https: //distributor.e-vuc.sk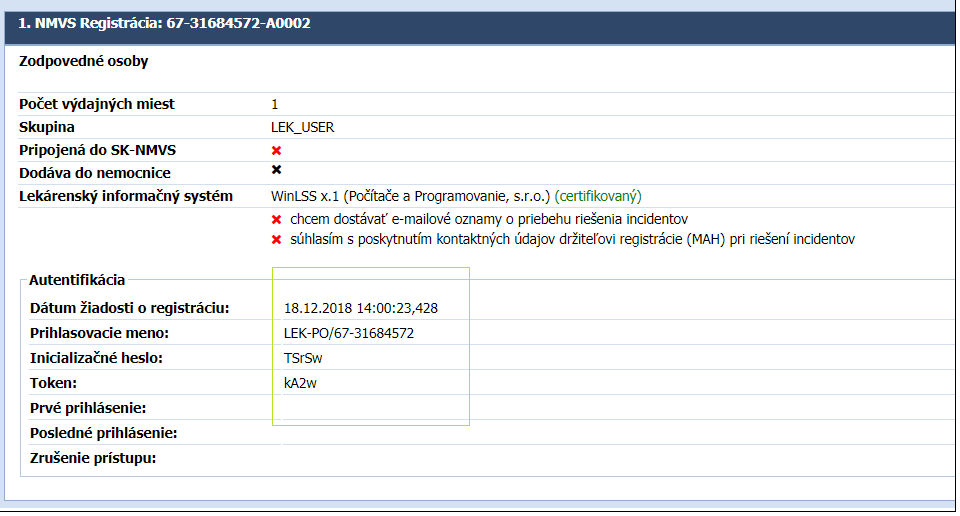
- After successfully connecting your system to the National Verification System, you can use the functionality of the verification of human medicines in your distribution company.

