On 9. On 1 February 2019, the European Union Delegated Regulation (EU) 2016/161 on falsified medicinal products, known as the FMD, came into force, making it mandatory to implement a European Union-wide medicines verification system. Slovakia was no exception and five years have passed since our pharmacists, after successful implementation of IT support systems, relevant changes to internal processes including relevant education, started to meet the requirements of the above legislation and to verify prescription medicines when dispensing them to patients.
Background information on FMD and its implementation
The European Union, through Directive 2001/83/EC of the European Parliament and of the Council of 6. November 2001 laying down the Community code relating to medicinal products for human use, established the legislative framework for the introduction of the so-called ‘Community code’. the safety features which must be fitted to the outer packaging of prescription medicinal products in order to identify each package of a medicinal product and thus distinguish it from another package of the same medicinal product and to make it easy to tell whether the outer packaging of a medicinal product has been tampered with. The specific technical specification of what the safety features are to look like and how they are to be used, how, when and by whom they are to be used to verify the packaging of a medicinal product and when these rules will apply to all EU Member States has been defined in the above-mentioned delegated FMD Regulation.
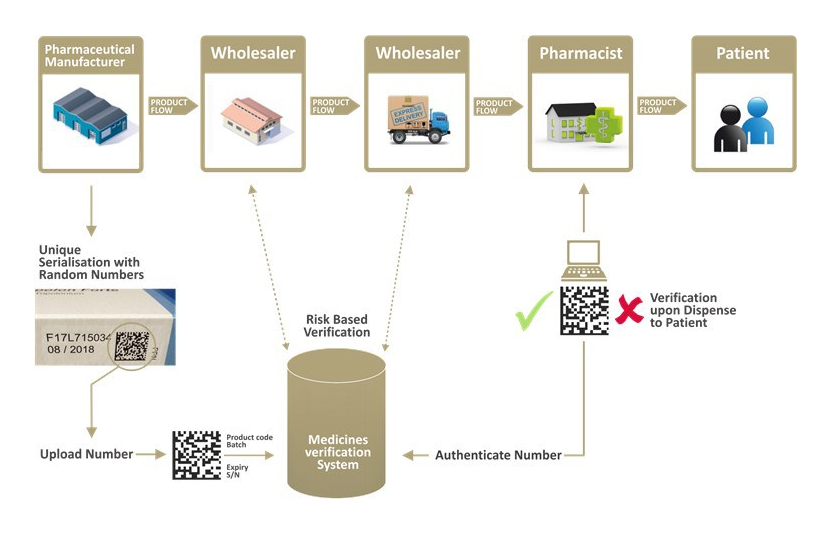
The thrust of the FMD legislation therefore lies mainly in the introduction of safety features that must be fitted to the outer packaging of the prescription medicine. There are two safety features, namely a device to identify the handling of the package – the so-called. an anti-tampering device and a unique specific package identifier that distinguishes one package of the medicine from another.
These elements make it possible to verify the packaging of the medicinal product when it is dispensed to the patient in terms of possible damage to the outer packaging of the medicinal product and also to verify that the specific identifier on the outer packaging of the medicinal product was indeed declared by the manufacturer to have been used in the manufacture of the packaging of the medicinal product and therefore manufactured by the manufacturer.
The specific identifier of the medicine package is placed on the outer packaging of the medicine package in two forms – in machine-readable form (a standardised 2D code defined by the GS1 standards organisation is used) and in human-readable form. Information on the specific identifiers of all prescription drug packages delivered into our legal prescription drug distribution chain is required to be uploaded by the manufacturer of the drug into our national verification registry, and this registry is used in accordance with the FMD by all entities that distribute and then dispense the drug package to the patient.

In accordance with the legislation in question, when dispensing a medicine pack to a patient, the pharmacy dispensing the medicine pack must deactivate the specific identifier of the dispensed medicine pack in the verification register, thereby indicating that the pack has been dispensed to the patient. A pharmacy shall be obliged to stop dispensing a medicinal product to a patient if the specific identifier indicated on the outer packaging of the dispensed package of the medicinal product is not present in the verification register or the specific identifier of that package is inactive.
A verification register is established in each EU country (except Italy and Greece, which have been granted a temporary derogation directly defined in the FMD) and all these national verification registers are interlinked. This allows pharmacies in Slovakia to verify prescription packs intended for several countries (multilingual packs) and also packs of medicines intended for another EU country but supplied to our market. In other words, a pharmacy in Slovakia can verify and also deactivate any pack of prescription medicine equipped with a specific identifier that is uploaded in any verification register of any EU country, and vice versa, any pharmacy in the EU can verify and deactivate a specific identifier stored in our verification register.
The described mechanism for the verification of medicinal products has been operational in Slovakia and throughout the EU since 9. February 2019.
Basic information about the Slovak Verification Register
The Slovak National Verification Register, its implementation, operation and maintenance is the responsibility of the Slovak Organization for Verification of Medicines – SOOL, which is a non-profit organization established by the associations of drug manufacturers, namely the Association of Innovative Pharmaceutical Industry – AIFP and the Association for Generic and Biosimilar Medicines – GENAS, in accordance with the requirements of the legislation. Other founding members are representatives of pharmacists represented by the Slovak Chamber of Pharmacists and distributors of medicines represented by the Association of Wholesale Distributors of Medicines AVEL. All of the named founders and members of SOOL shaped and oversaw the implementation of the verification registry and still have oversight of its operation.
The National Verification Register has been providing its services since its inception:
- 2 110 Slovak pharmacies and more than 115 000 pharmacies in other EU countries;
- 115 Slovak distributors of medicines and around 4 000 distributors from other EU countries;
- 320 manufacturers of medicines, represented by marketing authorisation holders (MAHs), who upload specific identifiers of prescription medicine packs manufactured by them and intended for the Slovak market to our register;
- In accordance with the legislation, ŠUKL is also a user of the register and has access to it.
In the 5 years since its inception, approximately 700 million specific identifiers of packages of medicines destined for our market have been uploaded to the National Verification Register. Pharmacies deactivate – and therefore verify – approximately 110 million packages of medicines per year. The registry is available 365 days a year, 24 hours a day, and responds to approximately 1 million requests from the above users every day. The average response time to a request is 30 milliseconds, which is well below the legislative requirement of 300 milliseconds.
Benefits of the Medicines Verification System after 5 years
The implementation of the FMD has significantly contributed to improving patient safety in Slovakia. Pharmacies that dispense medicines to patients now have greater assurance that the prescription medicines dispensed are authentic and have undergone rigorous verification. The system has also brought more transparency to the distribution processes of medicines. The whole pan-European medicines verification system, of which we are part, is an important preventive element which is essential to prevent counterfeiters from trying to get their falsified medicines into the official medicines distribution chain.
One of the important tangible results of the system is the interception of counterfeit packages of medicines – specifically packages of the oncology drug Imbruvica – in our legal drug distribution chain. On the basis of this fact, ŠUKL withdrew the entire batch of the medicine in question (in which the fake packages were found) from the market in 2021 (see information on the website www.sukl.sk dated 16.12.2021).
Challenges and further development of the medicines verification system
While FMD has brought many benefits, there are also challenges to be faced. These include the cost burden associated with the implementation of this legislation, both on the part of drug manufacturers and pharmacies, as well as on the part of drug distributors.
Another challenge is the unexpected number of technical incidents that are generated in the verification system and make life difficult for pharmacists and consequently for drug manufacturers. The highest ever number of incidents since the system was launched was recorded in the second week of April 2019, with more than 17,000 incidents in a week. The most common reasons for this are problems with the setup of package specific identifier readers in pharmacies, resulting in misinterpretation. In the first year of the launch of the verification system, an average of almost 8500 incidents caused by Slovak users were recorded per week. Fortunately, this has been rectified over the past two years, also in collaboration with more than 40 pharmacy and distribution IT system suppliers. By 2021 there was a significant decrease in the occurrence of incidents and by 2022 the numbers had stabilised. For comparison, in 2023 there were on average 330 incidents per week caused by Slovak users. Currently, Slovakia has the lowest error rate for reading specific identifiers in the EU.
Future perspectives include further development of technologies that can increase the efficiency of FMD, as well as other ways of using the system for the benefit of patients, such as the possibility of monitoring drug outages through the system. The fact that the packaging of a medicine is clearly identifiable opens the way for pharmacies and distributors alike to improve the management of the movement of the packaging of a medicine in their stock management. Here, the introduction of the so-called. aggregation functionality into the system. Of course, such options, if they go beyond the current legislation, must be supported by appropriate changes to that legislation.
Conclusion
The fifth anniversary of the implementation of the FMD in Slovakia is an appropriate opportunity to assess the positives and challenges of the implementation of this system. Patient safety, another important preventive barrier for counterfeiters, as well as more effective monitoring of the distribution of prescription medicines are key benefits of this system. The contribution of all stakeholders in the successful implementation and 5-year operation of this system should be highlighted. The approach of pharmacists to the above legislation should be highlighted, who, after initial mistrust of the functioning of such a system and after realising the usefulness of this element of patient protection, have adapted their internal processes in line with the legislation. Here, they have also been greatly helped by their IT pharmacy systems suppliers, who have implemented the functionalities necessary to meet the requirements of the legislation into these systems. However, thanks for the full functionality of the verification system in Slovakia should also be given to the representatives of the Ministry of Health of the Slovak Republic and the representatives of ŠÚKL, who assisted the successful implementation process, including the transformation of the FMD into our local legislation, with their methodological guidance and relevant guidelines. Thanks are also due to the distributors of medicines who, in particular, by agreeing to go beyond the FMD to check the packs supplied to pharmacies in order to prevent the distribution of packs of medicines that do not have their specific identifiers recorded in the verification register, have prevented many technical incidents in the system. Last but not least, the drug manufacturers who have borne and are bearing the greatest burden associated with the implementation and operation of this system should be highlighted. The coordination of the implementation and also the operation of the verification register is provided by the above mentioned SOOL organisation, which has managed and is managing this task to a good standard.
To meet the challenges and ensure a sustainable system in the fight against counterfeit medicines, there is a constant need to find innovative solutions and SOOL will continue to do so. However, this can only be done if there is an open dialogue between all stakeholders, including pharmacies, to continuously improve and adapt to the dynamics of the pharmaceutical industry, the dynamics of the drug distribution system and the rapidly evolving technological environment in order to meet the public’s expectation of the safety of the medicine chain.
Ing. Roman Guba

